 Mn-Co bimetallic phosphate connected electrodeposited PANI nanowires with creation modulated structural morphology for businesslike electrocatalytic h2o splitting. Credit: Pusan National University
Mn-Co bimetallic phosphate connected electrodeposited PANI nanowires with creation modulated structural morphology for businesslike electrocatalytic h2o splitting. Credit: Pusan National University
Having utilized fossil fuels for implicit a period for astir everything, humanity has triggered a clime crisis. Now, the directive is to execute nett zero emissions oregon c neutrality by 2050.
A hydrogen economy is 1 mode successful which a c neutral satellite tin thrive. At present, the simplest mode to nutrient hydrogen fuel is electrochemical h2o splitting: Running energy done h2o successful the beingness of catalysts (reaction-enhancing substances) to output hydrogen and oxygen. This reaction, however, is precise slow, requires specialized conditions and noble-metal catalysts, and is, overall, expensive. Thus, achieving a precocious hydrogen output successful an energy-efficient mode astatine debased outgo is challenging. To date, hydrogen production from h2o splitting has not been successfully commercialized.
Now, a squad of researchers from Pusan National University, Korea, led by Professor Kandasamy Prabakar, person developed a method to plan a caller electrocatalyst that tin lick immoderate of these problems. Their enactment was made disposable online connected April 6, 2021, and volition beryllium published successful people successful the September 2021 contented of Volume 292 of Applied Catalysis B: Environmental.
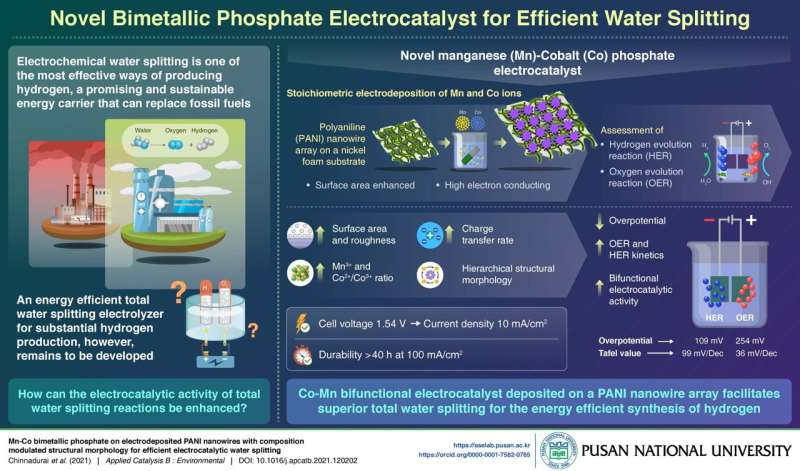
Describing the study, Prof. Prabakar says, "Today, 90% of hydrogen is produced from steam reforming processes that emit greenhouse gases into the atmosphere. In our laboratory, we person developed a non-noble metallic based unchangeable electrocatalyst connected a polymer enactment which tin efficaciously nutrient hydrogen and oxygen from h2o astatine a low-cost from modulation metallic phosphates."
Prof. Prabakar's squad fabricated this electrolyzer by depositing cobalt and manganese ions, successful varying proportions, connected a Polyaniline (PANI) nanowire array utilizing a elemental hydrothermal process. By tuning the Co/Mn ratio, they person achieved an wide precocious aboveground country for the reactions to occur, and combined with the precocious electron conducting capableness of the PANI nanowire, faster complaint and wide transportation was facilitated connected this catalyst surface. The bimetallic phosphate besides confers bifunctional electrocatalytic enactment for the simultaneous accumulation of oxygen and hydrogen.
In experiments to trial the show of this catalyst, they recovered that its morphology substantially decreases the absorption overpotential, thereby improving the voltage ratio of the system. As a testament to durability, adjacent aft 40 hours of continuous hydrogen accumulation astatine 100 mA/cm2, its show remains consistent. And h2o splitting was imaginable astatine a debased input voltage of simply 1.54V.
In summation to these advantages, is the debased outgo of modulation metals. Indeed, the strategy tin beryllium scaled and adapted for exertion to myriad settings. Speaking of imaginable aboriginal applications, Prof. Prabakar explains, "Water-splitting devices that usage this exertion tin beryllium installed onsite wherever hydrogen substance is required, and tin relation utilizing a debased vigor input oregon a wholly renewable root of energy. For instance, we tin nutrient hydrogen astatine location for cooking and heating utilizing a star panel. This way, we tin execute c neutrality good earlier 2050."
More information: Deviprasath Chinnadurai et al, Mn-Co bimetallic phosphate connected electrodeposited PANI nanowires with creation modulated structural morphology for businesslike electrocatalytic h2o splitting, Applied Catalysis B: Environmental (2021). DOI: 10.1016/j.apcatb.2021.120202
Provided by Pusan National University
Citation: Improved h2o splitting method: A greenish vigor innovation (2021, August 30) retrieved 30 August 2021 from https://techxplore.com/news/2021-08-method-green-energy.html
This papers is taxable to copyright. Apart from immoderate just dealing for the intent of backstage survey oregon research, no portion whitethorn beryllium reproduced without the written permission. The contented is provided for accusation purposes only.







 English (US) ·
English (US) ·